Silver Nitrate: A History
The use of silver in dentistry and homeopathic health is not new. In fact, SN has been used for several years in dentistry. (Fun Fact! The earliest known use of Silver Nitrate in a dental setting was in the year 1000 AD. Silver was first used in feudal Japan!) SN and its cousin, silver diamine fluoride, are commonly used to halt decay and the growth of cavities. Since we are a Fluoride Free office, we stick to the Nitrate and not the Silver Diamine Fluoride (SDF).
Silver Nitrate is most commonly used as an non-invasive treatment option especially in children or elderly.
What is Silver Nitrate?
Silver Nitrate (AgNO3) is a clear, odorless liquid that is astoundingly effective. The chemical finds, attaches to, and halts cavities (caries) in its path. The silver ions within the liquid have antimicrobial properties, and are naturally drawn to carious bacteria. The silver attaches and interacts with the bacterial DNA and cellular proteins almost instantaneously after application. As a result, bacteria can no longer survive once SN is applied. This leads to an immediate stop and halting of carious growth.
Another effect of AgNO3 is that it makes your teeth stronger. Dentin is the hard, bony tissue forming the bulk of your toot. The Dentin layer sits behind the Enamel layer and guards the blood vessels and nerves within the tooth itself. SN hardens carious, or cavity filled, dentin. Hardness can increase to the point where IT IS STRONGER THAN NATURAL HEALTHY DENTIN!
Does Silver Nitrate Work?
Yes! The ADA and its members have conducted several studies and independent experiments over the years. These studies show the effectiveness of SN in treatment and its ability to halt current and prevent future growth of existing cavities. A link to a 2013 study is found here.
https://www.ada.org/~/media/ADA/Education%20and%20Careers/Files/Bass_Hood_River_Symposium.pdf?la=en
Why don’t some dentists use Silver?
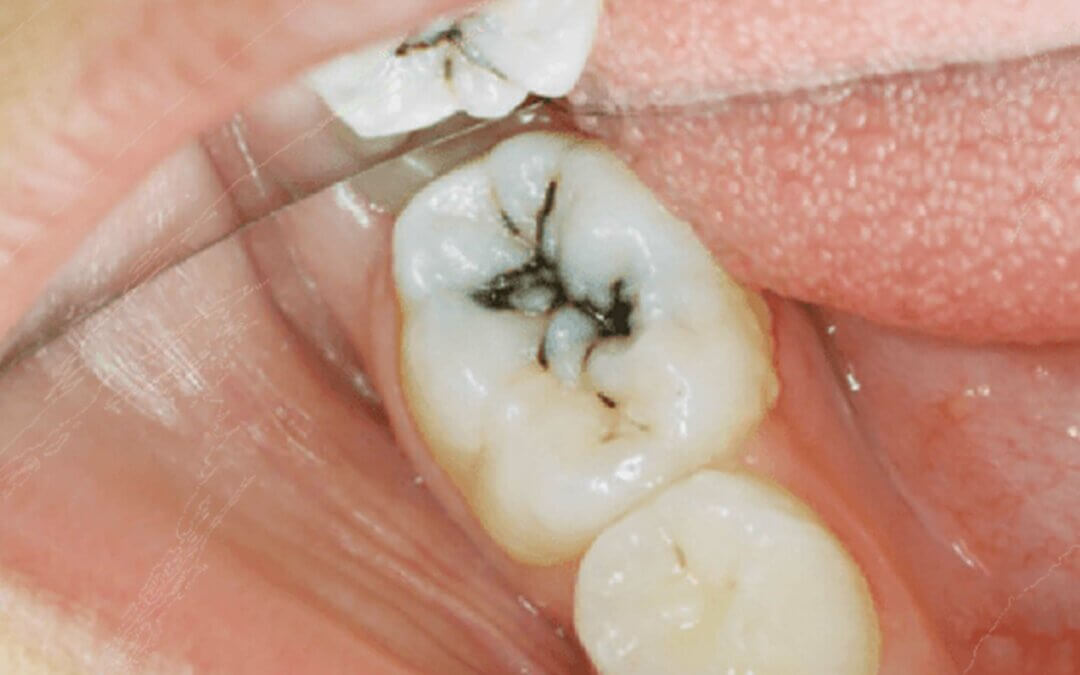
AgNO3 also has one defect. Reactions between silver ions and UV light turns the dentin & enamel layer a dark grey or blue-black color. This discoloration does not mean that your tooth is infected, it is the normal and expected reaction from application of Silver Nitrate on a cavity. Discoloration does not mean that AgNO3 should be eliminated from the office. Just like other Natural Medicines, every therapy has its place to be used safely and effectively.
How & When To Use SN Safely & Responsibly
As you can most likely guess, the discoloration caused by SN is an obvious detractor for any patient when it comes to using this type of therapy to halt carious growth. We recommend this alternative when faced with (but not limited to) the following scenarios.
- The patient is unwilling or unable to accept a filling as part of their standard treatment plan.
- A young child with primary teeth has cavities. The Cavities are primarily limited to the back teeth or are interior facing. The child or parents do not want to use under heavy sedation for the treatment.
- The patient requests Silver Nitrate as an effective alternative to fillings and stop cavities from growing larger.